What is a mixture? Let’s understand their types and their characteristics in detail.
Table of Contents
What is Mixture?
A mixture is a combination of two or more substances that are physically combined but not chemically bonded.
In a mixture, the individual substances retain their distinct properties and can be separated using physical methods. Mixtures can be made from various solids, liquids, or gases combinations.
Common examples of mixtures include air (a mixture of gases), saltwater (a mixture of salt and water), salad (a mixture of various ingredients), and concrete (a mixture of cement, sand, and gravel).
Characteristics Of A Mixture
- Physical Combination: A mixture is formed by physically combining two or more substances. The components are not chemically bonded and can be separated physically.
- Variable Composition: Mixtures can have varying proportions of their components. The ratio of substances in a mixture is not fixed and can change.
- No Chemical Reaction: No chemical reaction exists between the components in a mixture. Each substance in the mixture retains its chemical properties.
- Components Retain Properties: Each element in a mixture retains its chemical and physical properties. Each substance’s colour, density, boiling point, and other characteristics remain unchanged.
- Can Be Separated: The components of a mixture can be separated from each other using physical methods such as filtration, distillation, evaporation, or magnetism.
- No Fixed Ratio: Mixtures do not have a specific or fixed ratio of components. The proportion can vary depending on how the mixture is prepared or adjusted.
- Temporary: Mixtures can be temporary and quickly prepared and separated, allowing for flexibility in combining different substances for various purposes.
These characteristics make mixtures versatile and essential in chemistry, industry, and everyday life. Besides, they allow for creation of customized combinations of substances for specific purposes.
Types Of Mixtures
There are different types of mixtures because they result from various ways substances can combine or interact with each other.
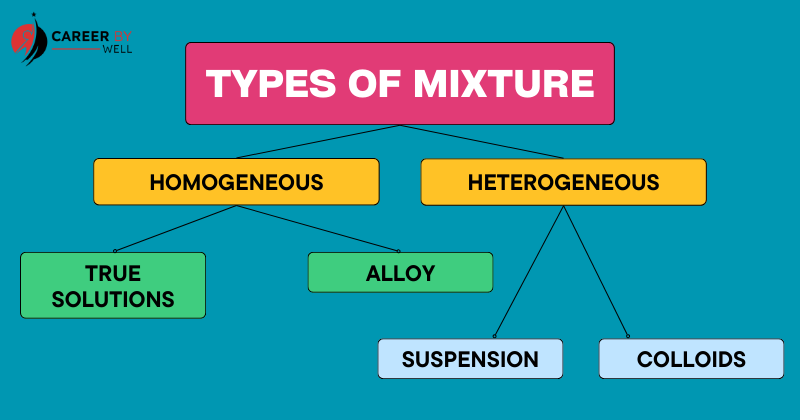
Further, the classification of mixtures is based on the degree of uniformity and interaction between the components. Usually, there are two types of mixture:
- Homogeneous Mixture
- Heterogeneous Mixture
Homogeneous Mixture
Homogeneous mixture is a mixure in which the components are uniformly distributed throughout the entire mixture.
A homogeneous mixture is also known as a solution. These types of mixtures have solutes and solvents. Together, they form a homogeneous mixture.
The individual substances, called solutes, are so thoroughly mixed with a solvent (the substance in which the solutes are dissolved) that the mixture appears as a single, consistent phase with a uniform composition.
Further, the physical and chemical properties of the components in a homogeneous mixture are the same throughout.
Examples: Common examples of homogeneous mixtures include saltwater (where salt is dissolved in water), sugar dissolved in tea or coffee, and air (a mixture of gases, primarily nitrogen and oxygen, that appears uniform to the naked eye).
Characteristics of Homogeneous Mixture
- Uniform Appearance: Homogeneous mixtures have a consistent and uniform appearance. There are no visible boundaries or distinct regions within the mixture.
- Components Indistinguishable: The mixture’s components (solutes) are thoroughly mixed and cannot be visually distinguished.
- Stable Mixture: Usually, homogeneous mixtures are stable, meaning they will remain uniform unless some external force or change in conditions, such as temperature, is applied.
- Solutes and Solvents: In a homogeneous mixture, the components are referred to as solutes (substances being dissolved) and solvents (the medium in which the solutes are dissolved). For example, salt is the solute in salt water, and water is the solvent.
Type Of Homogeneous Mixtures
- True Solution
- Alloy
1. True Solution
In chemistry, a true solution is a type of mixture in which a solute (usually a solid) is entirely and uniformly dissolved in a solvent (usually a liquid) at the molecular or atomic level.
These mixtures include saltwater (sodium chloride dissolved in water) and sugar dissolved in water.
In these cases, the solute particles (sodium ions and chloride ions in saltwater and sugar molecules in sugar solution) are dispersed at the molecular level in the solvent (water), creating a homogeneous and stable mixture with the characteristic features of a true solution.
Characteristics Of True Solution as Homogeneous Mixtures:
- Homogeneity: True solutions are perfectly homogeneous, meaning the solute particles are evenly distributed throughout the solvent. Besides this, uniform distribution occurs because the solute particles are molecular or atomic in size and are dispersed uniformly in the solvent, resulting in a single-phase mixture.
- Particle Size: In true solutions, the solute particles are typically smaller than 1 nanometer (nm) in size. This small particle size is responsible for the transparency and clarity of true solutions. Unlike suspensions or colloids, true solutions do not scatter light, making them optically clear.
- Stability: True solutions are stable and do not separate over time. Moreover, the solute remains uniformly dispersed in the solvent as long as no chemical or physical changes occur to disrupt the solution’s equilibrium.
- No Settling: Unlike suspensions, where larger particles can settle at the bottom over time, true solutions do not exhibit the settling of solute particles. The solute remains in a state of perpetual molecular dispersion.
- Filtration: True solutions cannot be separated from the solvent using simple filtration techniques because the solute particles are too small to be retained by filter paper or other porous materials.
- Transparent: True solutions are typically transparent and do not scatter light. Additionally, this transparency results from the solute particles being smaller than the wavelength of visible light, preventing the Tyndall effect (scattering of light) from occurring.
- Molecular Level Dissolution: In true solutions, the solute is completely dissolved at the molecular or atomic level, meaning that individual solute particles are dispersed as individual molecules or ions throughout the solvent. This results in a uniform and homogeneous mixture.
- Osmosis: True solutions exhibit osmosis, a phenomenon where solvent molecules move through a semipermeable membrane to equalize the concentration of solute particles on both sides of the membrane.
2. Alloy
An alloy is a mixture composed of two or more elements, typically metals, which are combined to form a homogeneous mixture with distinct characteristics.
Indeed, these characteristics make alloys necessary in various industries, such as engineering, manufacturing, and construction.
Usually, these mixtures offer a wide range of beneficial characteristics, such as improved properties, control over composition, and customization.
These features make alloys vital materials in various industries, where they are utilized for a diverse range of applications.
Characteristics of Alloys as Homogeneous Mixtures:
- Homogeneity: Alloys are created by mixing different elements at the atomic or molecular level. Besides, the atoms of the constituent elements are uniformly distributed throughout the alloy, resulting in a single-phase, homogeneous material. This means that the properties and composition of the alloy are consistent throughout.
- Improved Properties: Usually, alloys are engineered to have desirable properties superior to those of their component elements. For example, they can exhibit enhanced strength, hardness, corrosion resistance, electrical conductivity, or other attributes. By carefully selecting the elements and their proportions, engineers can tailor the properties of alloys to suit specific applications.
- Control over Properties: Generally, the composition of an alloy can be precisely controlled to achieve specific mechanical, thermal, or electrical properties. This control allows manufacturers to create materials with predictable and reliable characteristics, making alloys valuable for various industrial and technological applications.
- Customization: Alloys can be designed and customized to meet the specific requirements of a particular application. For instance, steel is an alloy of iron and carbon. Still, its properties can be adjusted by varying the carbon content and adding other elements.
- Alloy Families: There are numerous alloy families with unique elements and properties. These different alloy families offer a wide range of options for diverse applications.
- Versatility: Alloys find use in various industries, such as aerospace, automotive, and construction, due to their adaptability and versatility. Further, they make structural materials, electrical conductors, corrosion-resistant components, and many other specialized products.
- Corrosion Resistance: Alloys can be engineered to exhibit excellent resistance to corrosion, making them suitable for applications where exposure to moisture or corrosive substances is a concern.
- Economic and Environmental Benefits: Alloys often provide an economical solution compared to pure metals, as they can offer similar or superior performance while using less of the costlier metal.
Heterogeneous Mixture
A heterogeneous mixture is a types of mixtures in which the components are not uniformly distributed throughout the mixture.
A heterogeneous mixture is one of the types of mixtures that visually distinguishes the different components as they are not thoroughly mixed or blended.
Additionally, these components may exist in separate phases or regions within the mixture.
Common examples of heterogeneous mixtures include a salad (with separate components like lettuce, tomatoes, and croutons), a mixture of oil and water (with distinct layers), or a mixture of sand and gravel.
Characteristics Of Heterogeneous Mixture
Heterogeneous mixtures can have a wide range of appearances and properties. In many cases, the heterogeneity of a mixture is essential for specific processes or the desired outcome, such as in chemistry, cooking, or material science.
- Non-Uniform Appearance: Heterogeneous mixtures have an uneven and non-uniform appearance. Moreover, the mixture has visible boundaries or distinct regions where different components are concentrated.
- Components Distinguishable: The individual components in a heterogeneous mixture can be visually distinguished. They may appear as distinct phases, particles, or aggregates.
- Mixture Variability: Heterogeneous mixtures can vary in terms of the quantity and distribution of their components. Besides, the composition is not consistent throughout.
- Separation Possible: Because the components are not thoroughly mixed, it is often possible to physically separate them using methods like filtration, sedimentation, or decantation.
- No Solutes and Solvents: Unlike homogeneous mixtures (solutions) with solutes and solvents, heterogeneous mixtures do not follow this distinction. In a heterogeneous mixture, the focus is on the visible differentiation of components rather than on solvation.
Types Of Heterogeneous Mixtures
There are two types of mixtures in this category:
- Colloids
- Suspension
Colloids as Heterogeneous Mixtures:
A colloid is a of heterogeneous mixture where small particles of one substance are dispersed (spread out) evenly throughout another substance.
In these mixtures, the particles are larger than the individual molecules or ions in a solution but smaller than those in a suspension.
Additionally, the particles in a colloid do not settle out over time, and they remain suspended in the medium, giving the mixture a cloudy or milky appearance.
They are distinct from solutions, homogeneous mixtures, and suspensions, where larger particles settle over time. Colloids are important in fields where a stable and finely dispersed mixture of particles is desirable.
Common examples of colloids include milk (a colloidal suspension of fat droplets in water), mayonnaise, whipped cream, fog (a colloid of water droplets in air), and gelatin.
Characteristics of Colloids
- Particle Size: Colloidal particles are typically in the size range of 1 to 1,000 nanometers, larger than individual molecules but smaller than what is visible to the naked eye.
- Stable Dispersion: Colloidal particles remain stably dispersed in the medium (liquid, gas, or solid), and they do not settle to the bottom over time.
- Tyndall Effect: A colloid can scatter light, a phenomenon known as the Tyndall effect. When light passes through a colloidal solution, the path of the light becomes visible due to the scattering of light by the colloidal particles.
We will give details of Tyndall effects later in this part of the post.
Types of Colloids:
| Type of Colloid | Description | Example |
|---|---|---|
| Sol (Solid in Liquid) | In a sol, solid particles are dispersed within a liquid medium. The particles are typically small and remain stably dispersed. | Ink (carbon particles in water), blood (red blood cells in plasma) |
| Emulsion (Liquid in Liquid) | An emulsion consists of tiny liquid droplets suspended in another immiscible liquid. The two liquids do not readily mix. | Mayonnaise (oil droplets in water), vinaigrette (oil and vinegar) |
| Aerosol (Liquid or Solid in Gas) | Aerosols contain tiny liquid or solid particles dispersed in a gas. These particles remain suspended in the gas medium. | Fog (water droplets in the air), smoke (tiny solid particles in the air), and some inhalers (medication in a gas) |
Each type of colloid has specific characteristics and is important in various applications in science, industry, and daily life.
Suspension as Heterogeneous Mixtures
A suspension is a types of mixtures in which solid particles or liquid droplets are dispersed within a liquid or gas medium.
The particles in a suspension are typically larger and do not remain uniformly distributed throughout the medium.
Instead, they tend to settle at the bottom of the container over time due to the force of gravity, creating a visible boundary between the particles and the liquid or gas.
Examples: Common suspensions include a mixture of sand and water, orange juice with pulp, and certain medications that must be shaken before use.
Characteristics of Suspension
- Particle Size: Suspensions contain particles that are larger than those found in solutions and colloids. These particles can be seen with the naked eye.
- Non-Uniform Appearance: Suspensions have an uneven or non-uniform appearance. The particles settle at the bottom of the container, creating a distinct boundary between the solid or liquid and the surrounding medium.
- Separation Over Time: The particles in a suspension will settle out of the mixture when left undisturbed. Shaking or stirring the suspension may temporarily disperse the particles, but they will eventually settle again.
- Gravity’s Effect: The settling of particles in a suspension is primarily due to the force of gravity pulling them downward. This settling is often called sedimentation.
- Temporary Dispersal: While particles in a suspension can be temporarily dispersed by agitation, they will not remain evenly distributed for an extended period unless a stabilizing agent is added.
What is the Tyndall Effect?
The Tyndall effect is named after the 19th-century scientist John Tyndall. It is a phenomenon in which light is scattered or dispersed by small particles in a transparent medium.
This scattering of light causes the path of light to become visible. Then it produces a visible beam of light that appears as a cone or a halo.
Further, the Tyndall effect is particularly noticeable when light passes through a colloidal or suspension medium containing small particles, such as dust, droplets, or fine particles. Here are key points about the Tyndall effect:
Scattering of Light:
When light enters a medium with small particles, such as a colloid, the individual particles scatter the light in various directions. This scattering causes the light to become visible as it illuminates the particles’ paths.
Visible Path:
The paths of scattered light become visible to an observer, creating a visible beam or cone of light.
Further, the size and intensity of this effect depend on factors like the size of the particles, the concentration of particles, and the wavelength of the incident light.
Distinct from Solutions:
The Tyndall effect is a characteristic feature of colloids and suspensions but is not typically observed in solutions. In solutions, the particles are too small to scatter light significantly.
Applications:
The Tyndall effect has applications in various fields. It is used in industries to detect impurities in fluids, such as identifying the presence of particulate matter in liquids.
It is also employed in laboratory settings to study colloidal systems.
Common examples of the Tyndall effect include:
- I see a visible beam of light shining a flashlight or laser through a dusty room.
- The blue colour of the sky is due to the scattering of sunlight by atmospheric particles, demonstrating the Tyndall effect.
In short, the Tyndall effect is a visible scattering of light that occurs when light encounters small particles in a transparent medium. Thus it makes the light path visible and creates a visible beam of light.
This phenomenon is especially noticeable in colloids and suspensions containing particles large enough to scatter light effectively.
Final Thoughts
In short, the classification of mixtures into different types of mixtures reflects the diverse ways in which substances can interact and combine.
Further, these differences in particle size, homogeneity, stability, and other factors result in various mixture types with unique characteristics and applications.
Besides understanding these types of mixtures, it is essential in fields like chemistry, materials science, and various industries.