Elements or a substance can be classified into two categories. Metal and Non-metal. Both metal and non-metal have their own properties and characteristics. Based on this metal and non-metal have their own usage and advantages.
Elements or a substance can be classified into two categories. Metal and Non-metal. Both metal and non-metal have their properties and characteristics. Based on this, metal and non-metal have their usage and advantages.
What is Metal?
Metal is a chemical element class with specific properties and characteristics distinguishing them from nonmetals.
Metals are known for their lustre, electrical conductivity, thermal conductivity, malleability, and ductility. These properties make metals highly valuable and versatile materials in various industries and applications.
Critical characteristics of metals include:
Lustre:
Metals often have a shiny and reflective surface, known as metallic lustre. This property is due to the reflection of light by the free electrons in the metal’s atomic structure.
Electrical Conductivity:
Metals are excellent conductors of electricity. They contain a high density of free electrons that can move easily through the metal’s crystalline structure, allowing for the efficient flow of electric current.
Thermal Conductivity:
Metals also possess good thermal conductivity, meaning they can efficiently conduct heat. This property is valuable in applications where heat needs to be transferred or dissipated, such as in cooking utensils and heat exchangers.
Malleability:
Metals are malleable, which can be hammered, rolled, or pressed into various shapes without breaking. This property is essential in metalworking and manufacturing processes.
Ductility:
Generally, metals are ductile, which means they can be drawn into thin wires without breaking. Besides this property is crucial for applications like electrical wiring.
Solid State:
Usually, most metals are solid at room temperature, except mercury, a liquid at room temperature.
High Melting and Boiling Points:
Metals generally have high melting and boiling points, making them suitable for high-temperature applications.
Corrosion Resistance:
Some metals, such as aluminium and stainless steel, have natural corrosion resistance. Others may require protective coatings or alloys to prevent corrosion.
Examples of common metals include:
- Copper: Known for its electrical conductivity, copper is widely used in electrical wiring and as a component in various electronics.
- Iron: Iron and its alloys, such as steel, are extensively used in construction, manufacturing, and infrastructure.
- Aluminum: Aluminum is lightweight, corrosion-resistant, and widely used in aerospace, construction, and packaging.
- Gold and Silver: Precious metals like gold and silver have been valued for their rarity and beauty, making them important in jewellery and currency.
- Lead: While toxic, lead has historically been used in various applications, including piping, batteries, and radiation shielding.
Metals are fundamental in various industries, from construction and transportation to electronics and medicine. Their unique properties make them indispensable for various products and applications.
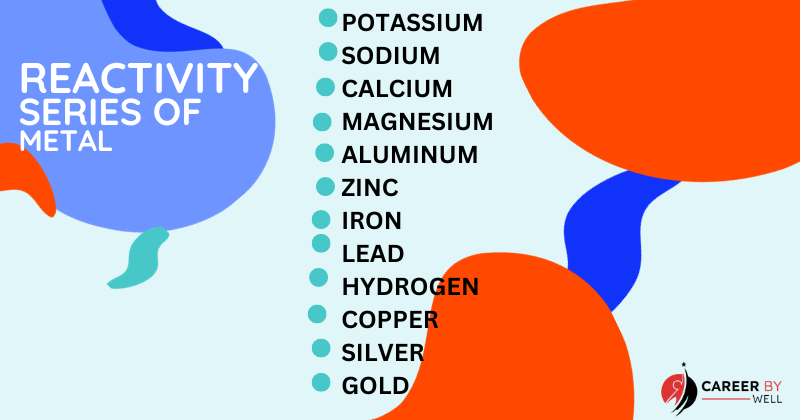
Exceptions Of Metals:
While the properties and characteristics described for metals generally hold true for most metallic elements, there are exceptions or unusual cases. Here are a few notable exceptions or deviations from typical metal properties:
- Mercury (Hg): Unlike most metals that are solid at room temperature, mercury is a liquid. It’s the only metal that is in a liquid state under standard conditions.
- Alkali Metals (Li, Na, K, Rb, Cs, Fr): The alkali metals, such as lithium (Li) and sodium (Na), are known for being highly reactive. They can even react vigorously with water, producing hydrogen gas and alkali hydroxides. This high reactivity sets them apart from many other metals.
- Alkaline Earth Metals (Be, Mg, Ca, Sr, Ba, Ra): Some alkaline earth metals like beryllium (Be) are toxic to humans and pose health hazards when inhaled or ingested. This contrasts with the general perception that metals are safe materials.
- Tin (Sn) and Lead (Pb): Tin and lead are known for their low melting points compared to other metals. Tin, for instance, can undergo a phase transition from a solid to a malleable “tin cry” state at very low temperatures.
- Bismuth (Bi): Bismuth is an example of a metal that expands as it solidifies rather than contracts, as most other materials do. This property makes it valuable in certain applications.
These exceptions demonstrate that while elements are grouped into categories of metal and non-metal on the periodic table based on their general properties, there is diversity and variation within each category.
Some elements exhibit unique or unexpected characteristics that deviate from the norms associated with their respective groups.
What is Non-metal?
Nonmetals are a class of chemical elements with properties and characteristics distinct from those of metals.
Unlike metals, nonmetals do not typically exhibit properties like metallic lustre, electrical conductivity, malleability, or ductility.
Instead, nonmetals often display insulating properties, low melting and boiling points, and the ability to gain electrons during chemical reactions.
Critical characteristics of nonmetals include:
- Lack of Metallic Luster: Nonmetals generally lack the shiny, metallic lustre that metals exhibit. Instead, they often have a dull or non-reflective appearance.
- Electrical Insulators: Most nonmetals are poor conductors of electricity. They have a limited number of free electrons that can move, which makes them insulators rather than conductors of electric current.
- Brittleness: Unlike metals, nonmetals are typically brittle and can shatter or break when subjected to stress or pressure.
- Low Melting and Boiling Points: Nonmetals generally have lower melting and boiling points than metals. They may exist in various states at room temperature, including solids (e.g., sulfur), liquids (e.g., bromine), and gases (e.g., nitrogen).
- Gain of Electrons: Nonmetals gain electrons during chemical reactions to achieve a stable electron configuration, forming negatively charged ions (anions).
- Chemical Reactivity: Nonmetals are often chemically reactive, participating in various chemical reactions, such as forming covalent compounds.
Examples of typical nonmetals include:
- Hydrogen: Although it is the lightest element, hydrogen is a nonmetal and exists as a diatomic gas (H2) at room temperature.
- Oxygen: Oxygen is essential for respiration and is a diatomic gas (O2) in its natural state.
- Nitrogen: Nitrogen is another diatomic gas (N2) that makes up a significant portion of the Earth’s atmosphere.
- Carbon: Carbon is known for its versatility and can exist in various forms, including diamond, graphite, and organic compounds.
- Sulfur: Sulfur is a nonmetal solid used to manufacture sulfuric acid and other chemicals at room temperature.
Nonmetals are critical elements in various chemical compounds and biological processes. They play a vital role in the structure of organic compounds, the atmosphere, and the chemistry of life.
They are often found in nonmetallic minerals and are used in applications ranging from plastics and semiconductors to environmental protection.
Exception Of Non-metal
Certainly, here are some exceptions or variations in the properties of nonmetals:
- Graphite (Carbon): While carbon is typically considered a nonmetal, graphite, one of its allotropes, conducts electricity. This is an exception to the usual nonmetal property of poor electrical conductivity. Graphite is used in applications like pencils and as a lubricant.
- Iodine: Iodine is a nonmetal exception to the rule of being a solid at room temperature. It sublimates directly from a solid to a vapour without melting, forming purple vapours.
- Selenium: Selenium is a nonmetal that can exhibit properties of both nonmetals and metalloids. It has semiconducting properties, making it useful in electronics.
- Hydrogen: Hydrogen is a nonmetal that can behave as both a nonmetal and a metal, depending on the conditions. Hydrogen can exhibit metallic properties, such as electrical conductivity, at high pressures.
- Bromine (Br): Bromine is a nonmetal, but it exists in a liquid state at room temperature and atmospheric pressure. This is unusual for nonmetals, typically in the gaseous or solid form at room temperature.
These exceptions highlight that nonmetals can display unique characteristics or properties under certain conditions or when arranged in specific forms or allotropes.
Difference Between Metal and Non-metal
These differences in properties and behaviour of metal and non-metal distinguish metals from nonmetals on the periodic table. They significantly affect their applications and roles in various chemical and industrial processes.
| Characteristic | Metals | Nonmetals |
|---|---|---|
| Appearance | Shiny (metallic luster) | Dull or non-reflective |
| Electrical Conductivity | Good conductors of electricity | Poor conductors (insulators) |
| Thermal Conductivity | Good thermal conductors | Poor thermal conductors |
| Malleability | Malleable (can be hammered) | Brittle (tend to break) |
| Ductility | Ductile (can be drawn into wires) | Not ductile (do not form wires) |
| Melting and Boiling Points | High melting and boiling points | Low melting and boiling points |
| Gain or Loss of Electrons | Tend to lose electrons (cations) | Tend to gain electrons (anions) |
| Chemical Reactivity | Tend to be less chemically reactive | Tend to be more chemically reactive |
| Location on Periodic Table | Located on the left and centre of the periodic table | Located on the right of the periodic table |
| Examples | Iron, copper, gold, aluminium | Hydrogen, Oxygen, Nitrogen, Sulfur |
What is Metalloid?
A metalloid, also known as a semimetal, is a class of chemical elements that exhibit properties and characteristics that are intermediate between those of metals and nonmetals.
Metalloids are positioned between metals and nonmetals on the periodic table and share some properties of both categories. They often display a combination of metallic and nonmetallic properties, making them distinct from pure metals and nonmetals.
Critical characteristics of metalloids include:
- Intermediate Electrical Conductivity: Metalloids can conduct electricity to some extent but are not as efficient conductors as pure metals. Their conductivity lies between that of metals and nonmetals.
- Variability in Appearance: Metalloids can have a variable appearance. Depending on the specific element and its form, they may appear metallic or nonmetallic.
- Semiconducting Properties: Many metalloids, such as silicon and germanium, are essential in electronics because they have semiconducting properties. This makes them valuable for the production of transistors and other semiconductor devices.
- Varying Chemical Behavior: Metalloids can exhibit varying chemical behaviour. Depending on the specific element and conditions, they may form covalent compounds like nonmetals or ionic compounds like metals.
Examples of common metalloids include:
- Silicon (Si): Silicon is a crucial component in manufacturing semiconductors and computer chips. It has both metallic and nonmetallic properties.
- Germanium (Ge): Like silicon, germanium is used in electronics, particularly in the production of transistors.
- Boron (B): Boron is found in compounds like borax and is used in various applications, including flame retardants.
- Arsenic (As): Arsenic is a toxic metalloid exhibiting metallic and nonmetallic properties in different chemical compounds.
- Antimony (Sb): Antimony produces certain alloys and flame-retardant materials.
- Tellurium (Te): Tellurium produces certain types of solar cells and is an essential component in some semiconductor materials.
Usually, metalloids play a vital role in various industries, including electronics, materials science, and energy production.
Further, their ability to conduct electricity under specific conditions and their semiconducting properties make them valuable in developing electronic devices and technologies.