Phase transition is a process in which one state of matter changes to another due to changes in temperature or pressure.
Phase transition is the process by which a substance transitions from one physical state to another due to changes in temperature or pressure.
Indeed phase transition phenomenon is governed by the kinetic theory of matter. This explains how the behaviour of particles (atoms or molecules) changes as they move from one state to another.
What is Matter?
Usually, Matter is the physical substance that makes up the universe and everything in it. Matter is composed of atoms, which are the smallest units of matter that retain the properties of an element.,
These atoms combine to form molecules, which can be simple. For example, H2O is the molecule for water or complex DNA, a large biomolecule.
Matter is anything which has mass and occupies space.
Indeed matter encompasses all substances in the universe that occupy space and have mass, and it can exist in various forms and states with distinct characteristics.
States of Matter
Matter can exist in various states or phases, including solid, liquid, gas, and plasma, depending on the arrangement and motion of its particles. Each state of matter has distinct physical properties and behaviours.
For example, solids have a fixed shape and volume, liquids have a definite volume but take the shape of their container, and gases have neither a fixed shape nor a fixed volume.
Principle Of Phase Transition
A phase transition, also known as a phase change, is a physical process in which a substance changes from one state of matter to another. There are different phase transition processes, such as
- Melting (Solid to Liquid)
- Freezing (Liquid to Solid)
- Vaporization (Liquid to Gas)
- Condensation (Gas to Liquid)
- Sublimation (Solid to Gas)
- Deposition (Gas to Solid)
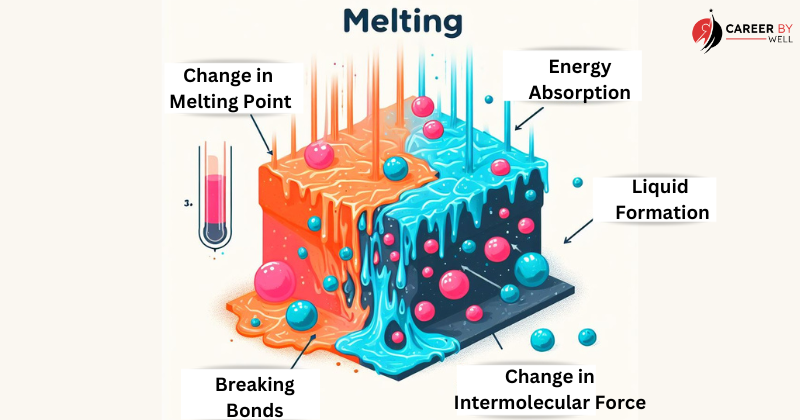
Melting (Solid to Liquid):
Melting occurs when a solid substance is heated, causing its particles to gain energy and move more rapidly.
As the temperature rises, the attractive forces between the particles weaken, allowing them to overcome the forces and move more freely.
This results in the solid turning into a liquid. The temperature at which this transition occurs is known as the melting point.
During the process of melting, these changes occur:
- Energy Absorption: As heat is added to the solid, the substance’s temperature rises. The added heat energy is absorbed by the particles (atoms, ions, or molecules) within the solid. This extra energy causes the particles to gain kinetic energy and move more rapidly.
- Overcoming Intermolecular Forces: In the solid state, particles are held together by intermolecular forces (e.g., van der Waals forces, hydrogen bonds) that keep them in a fixed, ordered arrangement. The particles must overcome these intermolecular forces to transition into the liquid state.
- Breaking Bonds: As the solid absorbs more heat energy, the particles gradually gain enough kinetic energy to break these intermolecular bonds or forces. This allows the particles to move more freely and independently of one another.
- Liquid Formation: Once enough energy is absorbed to overcome the intermolecular forces throughout the substance, the solid transforms into a liquid. The particles are still close together in the liquid state but move more randomly and do not have a fixed, ordered arrangement as in the solid state.
- Melting Point: The temperature at which melting occurs is known as the melting point or freezing point (for the reverse freezing process). Each substance’s melting point is a characteristic property and remains constant under specific pressure conditions.
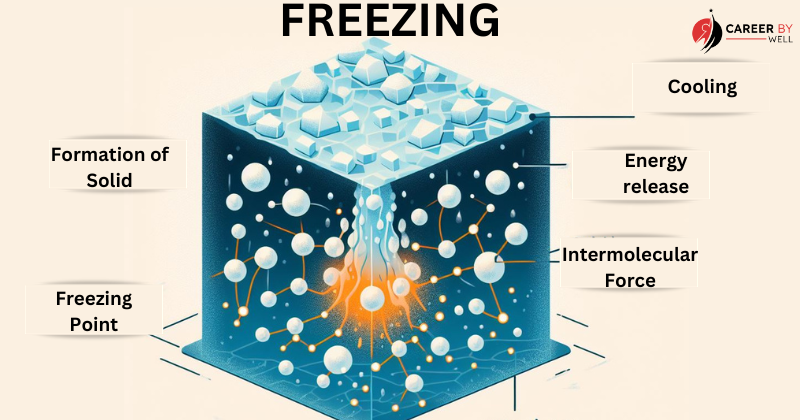
Freezing (Liquid to Solid):
Freezing is the reverse of melting. When a liquid is cooled, its particles lose energy and slow down.
As the temperature drops, the attractive forces between the particles become stronger, causing them to arrange themselves into a more ordered structure, forming a solid.
The temperature at which this occurs is the freezing point.
Here’s what happens during the process of freezing:
- Cooling: To initiate freezing, the liquid substance is exposed to lower temperatures. As it loses heat energy, its temperature gradually drops.
- Energy Release: As the temperature decreases, the particles (atoms, ions, or molecules) within the liquid lose kinetic energy and slow down. The excess energy is released in heat, which is why freezing is an exothermic process.
- Intermolecular Forces: In the liquid state, the particles are held together by intermolecular forces (e.g., van der Waals forces, hydrogen bonds). As the substance cools, these forces become stronger, causing the particles to become more closely packed and ordered.
- Formation of Solid Structure: When the temperature reaches the freezing point for that substance, the intermolecular forces are strong enough to lock the particles into a more stable, regular arrangement characteristic of a solid. This arrangement is usually a repeating crystalline structure.
- Freezing Point: The temperature at which freezing occurs is known as the freezing point. It is a specific temperature characteristic of each substance and remains constant under particular pressure conditions.
Vaporization (Liquid to Gas):
Vaporization is the process of a liquid turning into a gas. It can occur in two ways:
1. Evaporation:
This gradual process occurs at the surface of a liquid as particles gain enough energy to escape into the gas phase.
Evaporation happens at temperatures below the boiling point. Here’s how the process of evaporation works:
Process Of Evaporation:
- Heat Energy Absorption: The liquid substance absorbs heat energy from its surroundings through increased temperature or exposure to a heat source.
- Kinetic Energy Increase: As the substance absorbs heat, the kinetic energy of its particles increases. Particles move more rapidly and gain sufficient energy to break free from the liquid’s surface.
- Escape from Liquid: Some particles near the liquid’s surface gain enough energy to overcome the intermolecular forces (e.g., van der Waals forces) that hold them in the liquid. These particles escape into the air or surrounding space, becoming gas molecules or vapour.
- Continuous Process: Evaporation is an ongoing process that occurs at the liquid’s surface. It continues until a balance is reached between the rate of molecules escaping and the rate of molecules returning to the liquid (condensation). This balance is known as the equilibrium vapour pressure.
- Cooling Effect: Evaporation affects the remaining liquid because the particles with the highest kinetic energy leave the liquid, taking away energy from them. This is why sweating helps cool the human body.
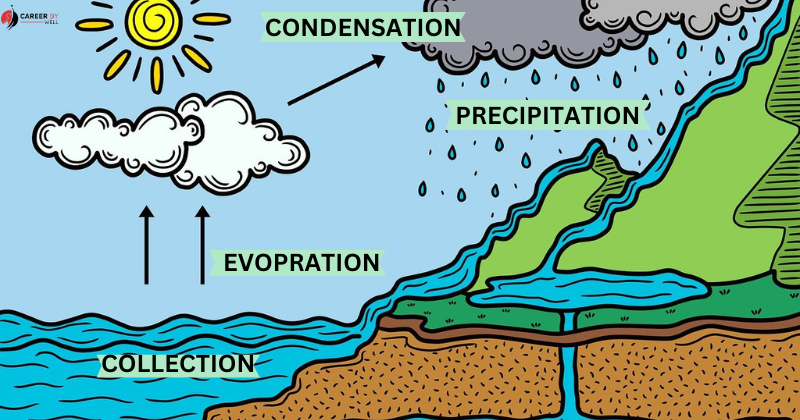
Evaporation is a critical natural process that plays a role in the water cycle, where water from the Earth’s surface (oceans, lakes, rivers) evaporates into the atmosphere, condenses to form clouds, and later falls back to the Earth’s surface as precipitation (rain or snow).
It also has practical applications in various industries, such as in the concentration of solutions, the production of salt, and in cooling systems like evaporative coolers and air conditioning.
2. Boiling:
Boiling is a more rapid process that occurs when the entire liquid reaches its boiling point, which is the temperature at which the vapour pressure of the liquid equals the atmospheric pressure.
This causes bubbles to form throughout the liquid, changing into a gas.
Process of Boiling
Here’s how the process of boiling works:
- Heating: Boiling typically begins when the liquid substance is heated. The liquid absorbs heat energy, causing the temperature to rise.
- Energy Absorption: As the liquid absorbs more heat energy, the kinetic energy of its particles (atoms or molecules) increases. Particles move more rapidly and gain sufficient energy to break the intermolecular forces that hold them together in the liquid state.
- Formation of Vapor Bubbles: When the temperature of the liquid reaches or exceeds its boiling point at a specific pressure, the intermolecular forces are overcome. This allows individual particles to escape the liquid’s surface and form gas or vapour bubbles within the liquid.
- Rapid Phase Transition: These vapour bubbles form throughout the entire volume of the liquid, not just at the surface. This rapid phase transition distinguishes boiling from other phases like evaporation, which occurs only at the liquid’s surface.
- Continuous Process: Boiling is ongoing as long as the heat source is applied and the temperature remains at or above the boiling point. New bubbles continually form, rise to the surface, and release vapour into the surrounding space.
- Boiling Point: The temperature at which boiling occurs is known as the boiling point. It is a characteristic property of each substance and varies with pressure. The boiling point for water at standard atmospheric pressure is 100 degrees Celsius (212 degrees Fahrenheit).
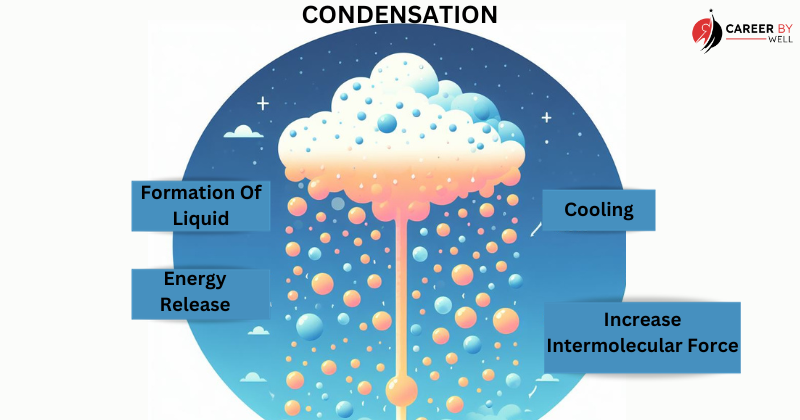
Condensation (Gas to Liquid):
Condensation is the reverse of vaporization. It occurs when a gas is cooled, causing its particles to lose energy and slow down. As the temperature drops, the gas particles form a liquid.
This process is responsible for forming clouds, dew, and fog. Here’s how condensation occurs:
- Cooling: Condensation typically happens when a gas or vapour is exposed to cooler temperatures or loses heat energy to its surroundings.
- Energy Release: As the gas particles lose energy, they slow down and reduce their kinetic energy. This excess energy is released into the surroundings, usually as heat.
- Attractive Forces Prevail: In the gas phase, particles are relatively far apart and have weak intermolecular forces. However, as the gas cools, the attractive forces between particles (e.g., van der Waals forces) become stronger.
- Formation of Liquid Droplets: When the temperature reaches a critical point known as the dew point, the attractive forces become strong enough to overcome the kinetic energy of the gas particles. This causes the gas particles to come together and form liquid droplets.
- Continuous Process: Condensation is a continuous process in which gas molecules continually transition to the liquid state as long as the cooling or energy loss persists. These liquid droplets may collect on surfaces, forming dew, fog, or clouds.
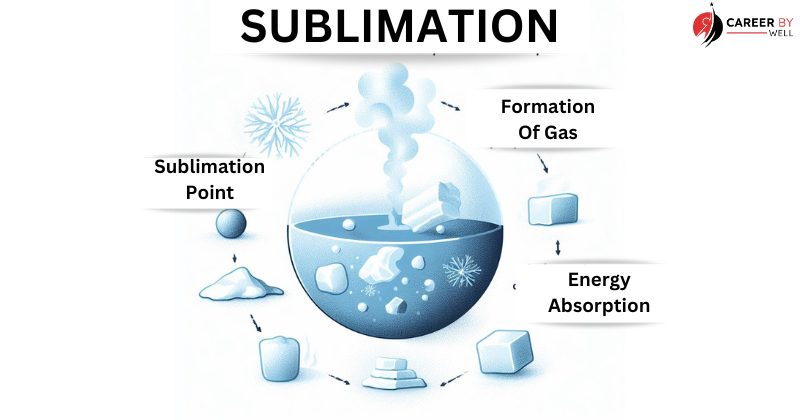
Sublimation (Solid to Gas):
Sublimation is the direct transition of a substance from a solid to a gas without passing through the liquid phase.
This occurs when a solid substance is heated, and its particles gain enough energy to move directly into the gas phase.
Common examples include dry ice (solid carbon dioxide) subliming into carbon dioxide gas and the process of freeze-drying. Here’s how sublimation works:
- Heating or Energy Absorption: Sublimation typically begins when the solid substance absorbs heat energy from its surroundings, usually due to increased temperature or exposure to a heat source.
- Energy Transfer: As the solid absorbs heat, its particles (atoms, molecules, or ions) gain kinetic energy and vibrate more vigorously. This increase in energy allows the particles to break free from their fixed positions in the solid lattice structure.
- Gas Formation: Instead of transitioning into a liquid state as in melting, the solid particles directly transition into a gas or vapour state. This means that solid particles become gas particles without going through liquefaction.
- Continuous Process: Sublimation is ongoing as long as the heat source is applied and the temperature remains at or above the substance’s sublimation point (sublimation temperature). The substance continually transforms from solid to gas without any liquid phase.
- Sublimation Point: The temperature at which sublimation occurs is known as the sublimation point or sublimation temperature. Like melting and boiling points, each substance’s sublimation point is a specific characteristic property.
Deposition (Gas to Solid):
Deposition is the reverse of sublimation. It happens when a gas changes directly into a solid without becoming a liquid first.
For example, water vapour in the air can undergo deposition to form frost or snow on a cold surface.
The behaviour of phase transition matter as it changes states is influenced by temperature, pressure, and the properties of the substance itself. Here’s how the process of deposition works:
- Cooling or Energy Release: Deposition typically begins when a gas or vapour loses heat energy to its surroundings. This cooling may occur due to a decrease in temperature or exposure to a cold surface.
- Energy Loss: As the gas particles lose energy, they slow down and reduce their kinetic energy. This reduction in energy causes the gas particles to come closer together.
- Formation of Solid Structure: When the temperature reaches the substance’s deposition point (the sublimation point in reverse), the gas particles have lost enough energy to overcome the intermolecular forces between them in the gas phase. These forces cause the particles to form a more ordered, solid structure.
- Solid Formation: The gas particles transition directly into a solid state and adhere to a surface or material, such as a cold object or the walls of a container. This results in the formation of solid deposits.
- Continuous Process: Deposition is ongoing as long as the cooling conditions persist and the temperature remains at or below the deposition point. It involves directly transforming gas particles into a solid without going through the liquid phase.
- Deposition Point: The temperature at which deposition occurs is the deposition point or deposition temperature. Like other phase transition points, it is a specific characteristic property of each substance.
Factors Affecting Phase Transition
Phase transitions, the processes in which a substance changes from one state of matter to another (e.g., solid to liquid or liquid to gas), are influenced by various factors. These factors can determine when and how a phase transition occurs. Here are the key factors that affect phase transitions:
Temperature:
Temperature is one of the most significant factors influencing phase transitions. Each substance has specific temperature points at which phase transitions occur, such as melting and boiling points.
Increasing the temperature generally promotes phase transitions by providing more thermal energy to the particles. This allows them to overcome intermolecular forces and change state.
Pressure:
Pressure plays a crucial role in phase transitions, particularly for multiple-phase substances. For example, depending on temperature and pressure conditions, water can exist as a solid, liquid, or gas.
Increasing pressure can alter phase transition points. For example, water’s boiling point increases at higher pressures.
Particle Interactions:
The nature and strength of interactions between particles (atoms or molecules) within a substance impact phase transitions.
Substances with strong intermolecular forces, such as hydrogen bonding in water, have higher melting and boiling points. Weaker forces make phase transitions occur more readily.
Purity:
The purity of a substance can affect its phase transition behaviour. Impurities or the presence of other substances can change the temperature at which a phase transition occurs.
For example, salt lowers the freezing point of water (used in winter road de-icing).
Particle Size:
The size of particles in a substance can influence phase transitions.
Smaller particles have a larger surface area and can exhibit phase transitions at slightly different temperatures than larger particles of the same substance.
Confinement:
The confinement of a substance within a container or structure can impact its phase transition behaviour.
For instance, a liquid in a small container may freeze or boil at a different temperature than the same liquid in a larger container.
Heating or Cooling Rate:
The rate at which a substance is heated or cooled can affect phase transitions.
Rapid cooling or heating may result in phase transitions at slightly different temperatures compared to slow, gradual changes in temperature.
Atmospheric Pressure:
The pressure of the surrounding atmosphere can influence phase transitions.
For example, at higher altitudes, the atmospheric pressure is lower, affecting water’s boiling point. Water boils at a lower temperature at higher altitudes because the reduced atmospheric pressure makes it easier for bubbles to form.
Presence of Catalysts:
Some substances, known as catalysts, can facilitate or enhance phase transitions. For instance, adding a catalyst can lower the activation energy required for a chemical reaction, which may sometimes affect phase transitions.
Crystallization Nuclei:
In the case of freezing or crystallization, tiny particles or nuclei can initiate the phase transition by providing surfaces for ice crystals to form. This is often seen in the process of freezing liquids like supercooled water.